Introduction
Periodontitis is a gram-negative anaerobic infection of the tooth-supporting structures characterized by chronic inflammation and immune reactions that result in loss of tooth supporting structures. This may be due to the direct action of periodontal pathogens or their products via transient bacteremia or indirectly through the acute phase response. The acute phase response is triggered by action of cytokines IL-1, IL-6 and TNF-α that act on the liver to produce acute phase proteins, altering the cellular and molecular blood chemistry. Three acute phase proteins of importance are C-reactive protein (CRP), Fibrinogen and serum Amyloid A.[1]
Fibrinogen is a type II, moderate, positive acute phase protein, produced in the liver and is often elevated in chronic inflammatory conditions.[1] It plays a vital role in coagulation process,blood viscosity, inflammation and atherogenesis.[2] Though secreted primarily by hepatocytes, extra hepatic secretion of fibrinogen by epithelial cells of lungs, intestine and human cervical epithelial cells has been reported in response to pro-inflammatory cytokines (IL-6).[2],[3] Gingival epithelial cells and fibroblasts in response to cytokines may also produce fibrinogen.[4] Excessive fibrinogen production can increase pro-inflammatory cytokines and attract more leukocytes at the sites of inflammation. Fibrinogen can also interact with bacteria promoting colonization and adhesion. Elevated fibrinogen levels can contribute reciprocally to inflammation further potentiating the inflammatory response. [4]
Fibrinogen is one of the important determinants of Erythrocyte sedimentation rate. ESR is a measure of an acute inflammatory response and ongoing coagulation process. Elevated ESR also alters the blood viscosity. [5],[6],[7] Evidence indicates that fibrinogen and ESR are intermediate risk factors for cardiovascular diseases.[8],[9],[10],[11] There is also evidence confirming the relationship between periodontal disease and cardiovascular disease.[12] Thus Fibrinogen and ESR may be possible systemic factors that may link periodontal disease and cardiovascular diseases.
Diseased periodontal tissues represent sites of localized activation of blood coagulation cascade.[4] However, involvement of blood clotting factors in the periodontal disease process has not been extensively investigated.It is possible that fibrinogen and ESR may serve as markers for the presence and severity of systemic inflammatory response in periodontitis patients.
The aim of this study was to evaluate whether periodontitis contributes to increased systemic inflammatory response reflected by elevated levels of fibrinogen & ESR.
Materials And Methods
Study Population
Subjects reporting to the Department of Periodontology, Vokkaligara Sangha Dental College and Hospital, Bengaluru, between October 2010 and April 2011 meeting the selection criteria were recruited for the study. The protocol for the study was approved by an institutional review board and all subjects signed a consent form to participate in the study.
Subjects were screened for suitability of the study based on the following criteria. Patients with age ranging from 25-55 years and with no systemic disease and history of blood transfusion within 4 weeks were included in the study. Also patients on any systemic drug, pregnant and lactating mothers, smokers and those who had undergone any periodontal treatment within six months of study were excluded from the study.
70 subjects (males & females) fulfilling these selection criteria were enrolled in the study.The subjects were categorized into three groups. Group I was the control group of 30 patients with healthy periodontium, no probable pocket and no signs of clinical attachment loss. Group II consisted of 20 patients with mild to moderate periodontitis, with clinical attachment loss of 1- 4 mm, in at least 6 teeth. Group III consisted of 20 patients with severe periodontitis, with clinical attachment loss ≥ 5 mm, in at least 6 teeth.
A detailed medical & dental history was compiled for all the subjects. Complete oral examination was carried out. The periodontal parameters included the assessment of Plaque index (Silness and Loe 1964), Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL) in all the groups. PPD and CAL were measured at 6 surfaces of each tooth using UNC -15 probe. Themeasurements were added and divided by six to obtain the mean PPD and CAL for the individual tooth.
Determination Of Fibrinogen, ESR and Lipid Parameters
8 ml of blood was drawn following overnight fasting from anti-cubital fossa by a clean veni- puncture using 20 gauge needles and a 10 ml syringe. 3 ml of blood was used for fibrinogen assay, 2ml for ESR and the other 3ml for lipid profile analysis.
(a).Measurement of plasma fibrinogen (FBG)
3ml of blood was transferred to a plastic vial containing 3.2% of buffered sodium citrate (anticoagulant) in the ratio of 9:1 and was mixed well. The specimen was then centrifuged at 1500x g for 15 minutes. Plasma was removed from the tube using a plastic pipette and was stored in a plastic tube at -70°C until analyzed. The specimens were thawed prior to analyses. Fibrinogen was assayed by Clauss method.
(b).Measurement of ESR
ESR was measured by Westergren method.
(c) Assessment of Lipid Parameters
Total Cholesterol, serum Triglycerides and high-density lipoprotein -Cholesterol were assessedusing A25 Biosystem auto analyzer. Other parameters assessed were VLDL, LDL & ratio of TC to HDL & LDL to HDL
Data obtained was subjected to statistical analysis
Statistical Analysis
Descriptive statistical analysis was carried out in the present study. Results on continuous measurements were presented as Mean ±SD (Min-Max) and results on categorical measurements were presented as Number (%). Significance was assessed at 5 % level of significance. Analysis of variance (ANOVA) was used to find the significance of study parameters between the three groups. Pearson correlation was used to establish the relationship between fibrinogen with ESR.
Results
Study Population
There was no statistically significant difference in the mean age, gender distribution and BMI & lipid parameters among the three groups indicating that the groups were matched with respect to these factors (p=0.961) (Table 1 & 2).
 | Table 1 : Showing Comparison of Age, gender and BMI in Group I, Group II and Group III.
 |
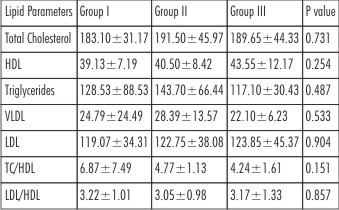 | Table 2 : Showing Comparisons of Lipid parameters in Group I, Group II and Group III.
 |
Periodontal Parameters
There was a statistically significant difference in the mean Plaque scores, Periodontal pocket depth & Clinical attachment level (CAL) between the groups (p< 0.001) (Table 3).
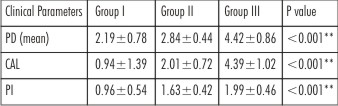 | Table 3 : Comparison of Clinical parameters pocket depth (PD), clinical attachment level (CAL) and Plaque index (PI) in Group I, Group II and Group III.
 |
Fibrinogen
Mean level of plasma fibrinogen in Group I was 296.90± 134.49 (SD), in Group II was 305.85± 63.64 (SD) and in Group III was 415.45± 171.90 (SD). The values showed gradual increase from control group (Group I) to mild moderate periodontitis group (Group II) to severe periodontitis (Group III) indicating that patients with moderate to severe periodontitis had higher mean levels of plasma fibrinogen than control group. The difference in values between Group I and Group II were not statistically significant (P=0.970). However, the difference in values between Group I and Group III and between Group II and Group III were highly statistically significant. Overall difference in levels of plasma fibrinogen were highly significant among groups (p<0.006) (Table 4).
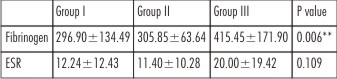 | Table 4 : Comparison of Fibrinogen and ESR in Group I, Group II and Group III
 |
Erythrocyte Sedimentation Rate (ESR)
Mean values for ESR was estimated as 12.24±12.43 (SD) for Group I, 11.40±10.28 (SD) for Group II and 20.00 ±19.42 (SD) for Group III. Values were numerically higher in Group I than in Group II and were highest in patients with severe periodontitis (Group III). However, there was no statistically significant difference in the ESR values among groups (p=0.109). (Table 4).
Correlation of fibrinogen with ESR
There was no statistically significant correlation of fibrinogen with ESR in all the three groups (Table 5).
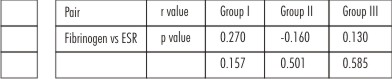 | Table 5
 |
Discussion
Periodontitis is a chronic inflammatory condition that results in elevated levels of haemostatic, lipid and protein markers.[1] Increased levels of these markers play a role in the development of cardiovascular diseases and may be associated with an increased risk of coronary events.[2] Several recent studies have emphasized that even moderately elevated levels of fibrinogen and ESR are predictors of increased cardiovascular risk even among apparently healthy individuals.[8],[9],[10],[11] Therefore this comparative study examined fibrinogen and the erythrocyte sedimentation rate as markers of systemic inflammatory response in patients with chronic periodontitis.
Fibrinogen levels have been reported to be influenced by age, gender, smoking, systemic diseases, inflammatory conditions like Rheumatoid arthritis, and concurrent use of antibiotics and anti-inflammatory agents.[2] Therefore such patients were excluded from the study.
The levels of fibrinogen are also influenced by BMI and lipid parameters. [2] Therefore, BMI and lipid parameters were assessed and all the three groups were matched with respect to age, gender, BMI and lipid parameters.
Plaque index, probing pocket depth and clinical attachment levels were recorded for all the subjects. There was a statistically significant difference in mean plaque scores among the three groups. Group I individuals showed good score (0.1-0.9), Group II individuals showed fair score (1- 1.9) and Group III individuals showed poor score (2-3). [13]
Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL)was measured using UNC-15 probe at mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual and distolingual surfaces of the tooth. All the six measurements were added and divided by number of sites examined to obtain the mean probing depth for the individual tooth. Clinical Attachment Level (CAL)measured as the distance from the cement-enamel junction (CEJ) to the base of the sulcus or the pocket is a better indicator of periodontal destruction. As expected periodontal parameters were significantly different in control, mild to moderate and severe periodontitis groups.[14]
Fibrinogen levels have been known to be influenced by intake of meal[2], therefore fasting blood samples were collected for all the patients. A variety of different tests and assays of @257;brinogen have been used by clinical laboratories. The assays vary in the degree of expertise and time required, and the equipment available. [15]
There are four methods for measuring fibrinogen levels which can be classified into two groups, ‘functional’ and ‘direct’. The first category involves tests based on the determination of the coagulation time, which in turn is proportional to the fibrinogen concentration. The most widely used method for the functional fibrinogen assay in most clinical laboratories is the Clauss method, which records the time taken to reach the coagulation end point (i.e. the formation of a clot).[15] The Clauss assays are generally reproducible but the normal reference interval must be determined for each laboratory for each assay, and is not a general value.[15]
This study measured fibrinogen levels using Von Clauss clotting method by a TriniClot fibrinogen kit (Trinity Biotech, Ireland). Reference value was established as 200-400 mg/dl. Reference curves were prepared at periodic intervals and measurements were made in duplicate on a log-log paper. Mean ± SD for fibrinogen in Group I was 296.90±134.49, in Group II was 305.85±63.64 and in Group III was 415.45±171.90. The values showed gradual increase from control patients (Group I) to moderate periodontitis group (Group II) with a marked difference in the values from moderate to severe periodontitis (Group III). The gradual increase in values of plasma fibrinogen from control group, to moderate periodontitis group and with maximum values in severe periodontitis group supports the evidence of plasma fibrinogen as a marker for the presence and severity of systemic inflammatory response in periodontitis patients. This is consistent with other observational studies.[4],[16],[17],[18],[19],[20]
A statistically significant difference in fibrinogen values between healthy controls and periodontitis patients has been reported by Bizarro, et al (2005).[16] Similarly a significant association between indicators of poor periodontal status and increased fibrinogen levels has been reported by Wu, et al (2000).[17] A meta-analysis by Danish, et al (1998) reported a significant association of elevated fibrinogen levels in periodontitis patients with a subsequent risk of cardiovascular diseases.[18] Higher levels of fibrinogen has been reported in patients with periodontitis and 455G/A- fibrinogen gene polymorphism.[4] However, a cross-sectional study by Joshipura J, et al (2004) did not report a statistically significant association in levels of plasma fibrinogen and self reported periodontal disease.[21]
Effects on Fibrinogen levels in the plasma as a measure for a decrease in systemic inflammatory response after treatments like full mouth extraction [22] and non-surgical therapy [23], [24] have also been evaluated but with conflicting results.
Fibrinogen differs from other acute-phase proteins in its ability to be constitutively expressed at a moderate level.[2] During an acute-phase response, this effect can be initiated by IL-6, inflammatory mediators like PGE2, as well as by lipopolysaccharides from various periodontal pathogens. Additionally although, the liver is the primary site of @257;brinogen (FBG) synthesis, epithelial cells from diverse tissues like pulmonary, intestinal and cervical cancer have been reported to respond to local in@258;ammation by increased transcription of the γ-FBG gene.[2],[3],[4] It is possible thatsimilar process may be responsible for local production of FBG from gingival epithelium and fibroblast and thus contribute to significant increase in systemic plasma fibrinogen levels in severe periodontitis patients.[4]
Fibrinogen also plays a critical role in exacerbation of inflammatory periodontal disease.[4] Elevated fibrinogen levels interact with periodontal bacteria favouring their colonization and adhesion.[4] Also fibrinogen favours increased leukocyte recruitment and cytokine production at the site of infection as evidenced by positive correlation between increased levels of fibrinogen and the number of leukocytes.[2] Fibrinogen serves as a native ligand for CD11b/CD18 surface receptors of polymorphonuclear leukocytes (PMNs) (Walzog et al, 2000). [4]
Fibrinogen also reciprocally contributes to inflammation through facilitating a chemotactic response by binding to its integrin receptor (MAC-1) on leukocytes (macrophages and myelocyte). This results in differential expression of ‘proinflammatory’ cytokines. Interaction with the cytokines results in conformational changes within the fibrinogen molecule and conversion into ‘proinflammatory’ fibrinogen, resulting in the exposure of the epitope that interacts with the MAC-1 receptor for macrophages.[2]
There is evidence for a functional link between clotting and inflammatory responses. Fibrinogen has been found to enhance the adhesion of cells of myeloid and lymphoid lineage to endothelium by 2- to 5-fold by binding to intercellular adhesion molecule-1 (ICAM-1). Authors also demonstrated that fibrinogen could regulate expression of the IL-1 beta gene, causing increased IL-lβ production both at the mRNA and protein level.[4] IL-1 has a central role in periodontal tissue destruction.[26]
The reported value of fibrinogen as a risk factor for cardiovascular diseases is 286 mg/dl by Clauss method; 57.1% of the subjects in our study had plasma fibrinogen levels higher than 286mg/dl. 40% of subjects in Group I, 60% in Group II and 80% in Group III exhibited values above this level. These values could contribute in part to increased risk of cardiovascular diseases.[2]
The ESR is a traditional parameter of any given inflammatory process.[5],[6],[7] It provides a multifactorial measure of the systemic response to an infectious or inflammatory disease. Fibrinogen levels affect the erythrocytes rouleaux formation and subsequently precipitation (sedimentation).[5],[6],[7]
ESR was assessed by Westergren method which is a standard method as recommended by International Committee for Standardization in Hematology (ICSH).[27] Mean values for ESR for Group I were 12.24±12.43 (SD), for Group II were 11.40±10.28 (SD) and for Group III were 20.00±19.42 (SD). Values were numerically higher in Group I than in Group II and were highest in patients with severe periodontitis (Group III). However, there was no statistically significant difference in the ESR values among the three groups. The results of the present study are in accordance with earlier studies in which measurement of ESR as a diagnostic tool for periodontitis was assessed. ESR was found to be of only limited significance.[28]
Although fibrinogen is an important determinant of ESR[5], no correlation could be established between the fibrinogen and ESR values possibly because hematocrit and other non-fibrinogen proteins also influence ESR.[6], [7]
CRP is considered as a diagnostic tool for assessment of systemic inflammatory response.[29] However, for cardiovascular diseases which are haemostatic as well as rheologically influenced diseases fibrinogens holds greater importance as its values give greater insight on the inflammatory, thrombotic and atherosclerotic status of an individual. Longitudinal studies suggest that fibrinogen and ESR are intermediate risk factors for cardiovascular diseases like atherosclerosis, myocardial infarction and stroke.[8],[9],[10],[11],[12] A recent study showed that elevated ESR was a strongest and most significant predictor of risk of cardiovascular disease among several plasma variables.[11] Although periodontal disease is not established as causal factor for CHD, observational studies establish it as an independent definitive risk factor for CVD.[30],[31] Therefore elevated levels of fibrinogen and ESR serve as a marker of presence and severity of systemic inflammatory response in patients with periodontitis and may be possible intermediate factors that may link periodontal disease and cardiovascular diseases.
Conclusion
Although significance of ESR as a marker of presence and severity of systemic inflammatory response was limited, fibrinogen served as a definite marker of systemic inflammatory response in chronic periodontitis. As cardiovascular diseasesare haemostatic as well as rheologically influenced diseases fibrinogen holds greater importance than other variables in linking possible risk for cardiovascular diseases.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a finan¬cial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Acknowledgments
I acknowledge Mr Sridhar, Manager and Mr Azim, Senior lab technician of Chanre diagnostics, Malleshwaram, Bengaluru. I thank Dr Anjana Gopi, Associate professor, Department of Microbiology, Kempegowda Institute of Medical Sciences, Bengaluru for her help and co-operation. Special thanks to Dr. Arvind Kavishwar, Biostatistician, for helping me with the statistical analysis.
References
1. Ebersole JL, Cappelli D. Acute-phase reactants in infections and in@258;ammatory diseases. Periodontol 2000, 2000; 23: 19–49.
2. Kamath S, Lip G. Fibrinogen: biochemistry, epidemiology and determinants. Q J Med 2003; 96: 711–729.
3. Haidaris P. Induction of fibrinogen biosynthesis and secretion from cultured pulmonary epithelial cells. Blood 1997; 89: 873-882.
4. Sahingur S, Sharma A, Genco R, Ernesto D, et al. Association of increased levels of fibrinogen and the -455G/A fibrinogen gene polymorphism with chronic periodontitits. J Periodontol 2003;74: 329-337.
5. Jurad RL. Why shouldn’t we determine the erythrocyte sedimentation rate? CID 2001; 33: 548-549.
6. Means RT, Glader B. Clotting mechanism. Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Arber DA et al.(ed) Wintrobe's Clinical Hematology, 12th Edition. Philadelphia Lippincott Williams & Wilkins Publishers, 2009.1144-1159.
7. Steinvil A, Shapira I, Arbel Y, Justo D, Berliner S, Rogowski O, et al. Determinants of the erythrocyte sedimentation rate in the era of micro-inflammation. Am J Clin Pathol 2008; 129: 486- 484.
8. Stone MC, Thorpe JM. Plasma fibrinogen – a major cardiovascular risk factor. J Royal Collg Gn Pract 1985; 35: 565-569.
9. Meade TW. Fibrinogen in ischaemic heart disease. J Clin Path 1997; 50: 13-15.
10. Ernst E, Ludwig K. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Int Med. 1993; 118: 956-963.
11. Erikssen G, Liestol L, Bjornholt J, Stormorke H, Thaulow E, Erikssen J. Erythrocyte sedimentation rate: a possible marker of atherosclerosis and a strong predictor of coronary heart disease mortality. Eur Heart J 2000; 2: 1614–1620.
12. Ouyng XY, Xiao WM, Chu Y, Zhou S. Influence of periodontal interventional therapy on risk of cardiovascular disease. Periodontol 2000, 2011; 56: 227-257.
13. Soben Peter. Indices in dental epidemiology. Essentials of preventive and community dentistry. 2nd edition. India. Arya publishers, 2003. 127-240.
14. Newman MG, Takie HH, Klokkevold PR, Fermin AC. Chronic Periodontitis. Forest JN, Hujoel PP, Lieberman MB. (ed) Carranza’s Clinical Periodontology. 10th edition. India. Elsiever Publishers, 2007. 494-499.
15. Mackie I, Kitchen S, Machin S,Lowe GD. Guidelines on fibrinogen assay. Brit J Hema 2003; 121: 396-404Gruys E, Toussaint M, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ SCI 2005; 6(11): 1045-1056.
16. Bizarro S, Velden U, Leivadaros E, et al. Markers of co-agulation and fibrinolysis in periodontitis. J Dent Res 2005; 84:122-126.
17. Wu T, Trevisan M, Genco R, Falkner K, Dorn J, Christopher T. Examination of the relation between periodontal health status and cardiovascular risk factors: Serum total cholesterol and high density lipoprotein cholesterol, C- reactive protein, and plasma fibrinogen. Am J Epidemiol 2000; 151: 273-282.
18. Danish J, Collins R, Appleby P, Peto R, et al. Association of fibrinogen C-reactive protein, albumin or leukocyte count with coronary heart disease: Meta-analysis of prospective studies. J Am Med Assoc 1998; 279: 1477-1482.
19. Kweider M, Lowe GD, Murray GD, Kinane DF, McGowan DA. Dental disease, fibrinogen and white cell count, links with myocardial infarction? Scot Med J 1993; 38: 73-74.
20. Jukka H, Meurman D, Janket S, Qvarnstrom S, Nuutinen P. Dental infections and serum inflammatory markers in patients with and without severe heart disease. Oral Surg Oral Med Oral Path Oral Rad End. 2003; 96(6): 695-700.
21. Joshipura J, Wand H, Merchant A, Rimm E, et al. Periodontal disease and biomarkers related to cardiovascular disease. J Dent Res 2004; 83(2): 151-155.
22. Reinhart WH. Fibrinogen - marker or mediator of vascular disease? Vasc Med 2003; 8: 211-216.
23. Taylor BA, Tofler GH, Carey HM, Morel MC, Philcox S, Carter TR, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res 2006; 85: 74-80.
24. Ide M, Mcpartlin M, Coward P, Crook M, Lumb P, Wilson RF. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol 2003; 30: 334–340.
25. Montebugnoli L, Servidio D, Miaton RA, Prati C, Tricoci P, Melloni C, et al. Periodontal health improves systemic inflammatory and haemostatic status in subjects with coronary heart disease. J Clin Periodontol 2005; 32 (2): 188 – 192.
26. Newman MG, Takie HH, Klokkevold PR, Fermin AC. Microbial interaction with the host in periodontal disease. Forest JN, Hujoel PP, Lieberman MB (ed). Carranza’s Clinical Periodontology. 10th edition. India. Elsiever Publishers, 2007. 228-250.
27. Saadeh C. The erythrocyte sedimentation rate: old and new clinical applications. J Clin Path 1993; 48: 210-215.
28. Aggrawal N, Veerendra SC, Gujjari A. Effect of periodontal therapy on haemoglobin and erythrocytes levels in chronic generalised periodontitis patients: an interventional study. J Ind Soc Periodontol 2009; 13(1): 6-11.
29. Loos B. Systemic markers of inflammation in periodontitis. J Periodontol 2005; 76: 2106-2115.
30. Genco R, Steven, Offenbacher, Beck J, et al. Periodontal disease and cardiovascular diseases. J Am Dent Assoc 2002; 133: 145-225.
31. Humphrey L, Fu R, Buckley D, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Inter Med 2008; 23(12): 2079–2086.
|